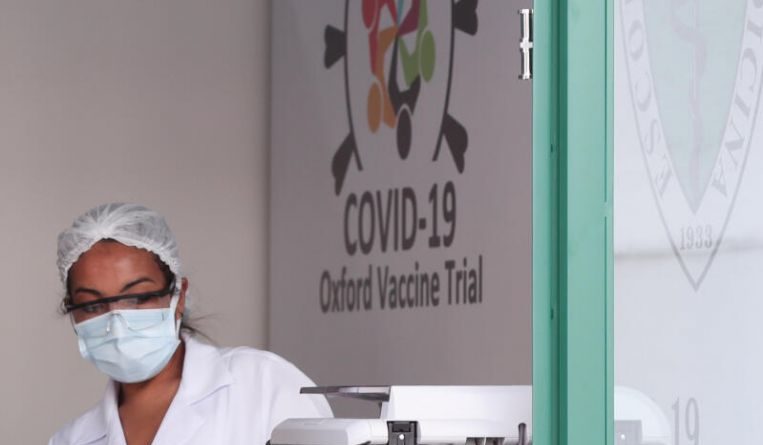
COVID-19 PANDEMIC: THE CURE-VACCINE- Over 1 million doses of Oxford-AstraZeneca Covid-19 vaccine possible by September
“The 2019 Plague” Day 235
.
LONDON (REUTERS) – Early estimates of the production of a million doses of the University of Oxford’s experimental Covid-19 vaccine by September could be an underestimate depending on how quickly late-stage trials can be completed, a researcher said on Monday (July 20).
“There might be a million doses manufactured by September: that now seems like a remarkable underestimate, given the scale of what’s going on,” Adrian Hill of University of Oxford said, referring to the manufacturing capability of partner AstraZeneca.
.


.
“Certainly there’ll be a million doses around in September. What’s less predictable than the manufacturing scale-up is the incidence of disease, so when there’ll be an endpoint.”
He added it was possible that there would be vaccines available by the end of the year.
Earlier, AstraZeneca reported that its experimental Covid-19 vaccine was safe and produced an immune response in early-stage clinical trials in healthy volunteers, its data showed on Monday, with the strongest response seen in people who received two doses.
The vaccine, called AZD1222 and being developed by AstraZeneca and scientists at Britain’s University of Oxford, did not prompt any serious side effects and elicited antibody and T-cell immune responses, according to trial results published in The Lancet medical journal.
“There is still much work to be done before we can confirm if our vaccine will help manage the Covid-19 pandemic, but these early results hold promise,” vaccine developer Sarah Gilbert said.
“We still do not know how strong an immune response we need to provoke to effectively protect against SARS-CoV-2 infection.” Gilbert said researchers needed to learn more about Covid-19 and continue late stage trials which have already commenced.
British Prime Minister Boris Johnson described the early stage clinical trial data as very positive on Monday.
“This is very positive news. A huge well done to our brilliant, world-leading scientists & researchers at #UniofOxford,” Johnson said on Twitter, linking to a report on the data.
“There are no guarantees, we’re not there yet & further trials will be necessary – but this is an important step in the right direction.”


.
AstraZeneca shares spiked higher, but then gave up some gains, to last trade up 0.4% on the day.
AstraZeneca’s is among the leading vaccine candidates against a pandemic that has claimed more than 600,000 lives, alongside others in mid and late-stage trials.
These include shots being developed by China’s Sinovac Biotech, another from state-owned Chinese firm Sinopharm, and one from the US biotech firm Moderna.
AstraZeneca has signed agreements with governments around the world to supply the vaccine should it prove effective and gain regulatory approval. The company has said it will not seek to profit from the vaccine during the pandemic.
Researchers said the vaccine caused minor side effects more frequently than a control group, but some of these could be reduced by taking paracetamol, with no serious adverse events from the vaccine.


.
AZD1222 was developed by Oxford university and licensed to AstraZeneca, which has put it into large-scale, late-stage trials to test its efficacy. It has also already signed deals to produce and supply over 2 billion doses of the shot.
The new trial included 1,077 healthy adults aged 18-55 years with no history of Covid-19.
“Today’s data increases our confidence that the vaccine will work and allows us to continue our plans to manufacture the vaccine at scale for broad and equitable access around the world,” said Mene Pangalos, Executive Vice President of BioPharmaceuticals Research and Development at AstraZeneca.
Moderna, another front runner, released results last week from an early-stage test that showed its vaccine raised levels of antibodies that fight the virus.


.
Read the latest on the Covid-19 situation in Singapore and beyond on our dedicated site here.
Get The Straits Times app and receive breaking news alerts and more. Download from the Apple App Store or Google Play Store now.